
Jun 06, 2022 / Author: China Glutathione suppliers & NMN manufacturers
On April 11, 2022, Japan completed a placebo-controlled, randomized, double-blind, parallel-group trial to investigate the safety of oral NMN and its efficacy in increasing NAD+ levels in 30 healthy subjects .The following is the original text of the experimental discussion:
The present study investigated the safety of orally administered NMN in healthy participants. We also examined the NAD+ metabolism and amino acid metabolism during NMN administration. We demonstrated that taking 250 mg of NMN every day for 12 weeks is a safe and well-tolerated practice in healthy individuals. In addition, the NAD+ levels in whole blood were significantly increased in the NMN-treated group. These results suggest that the oral administration of NMN is safe and can be a practical strategy to boost NAD+ levels in humans.
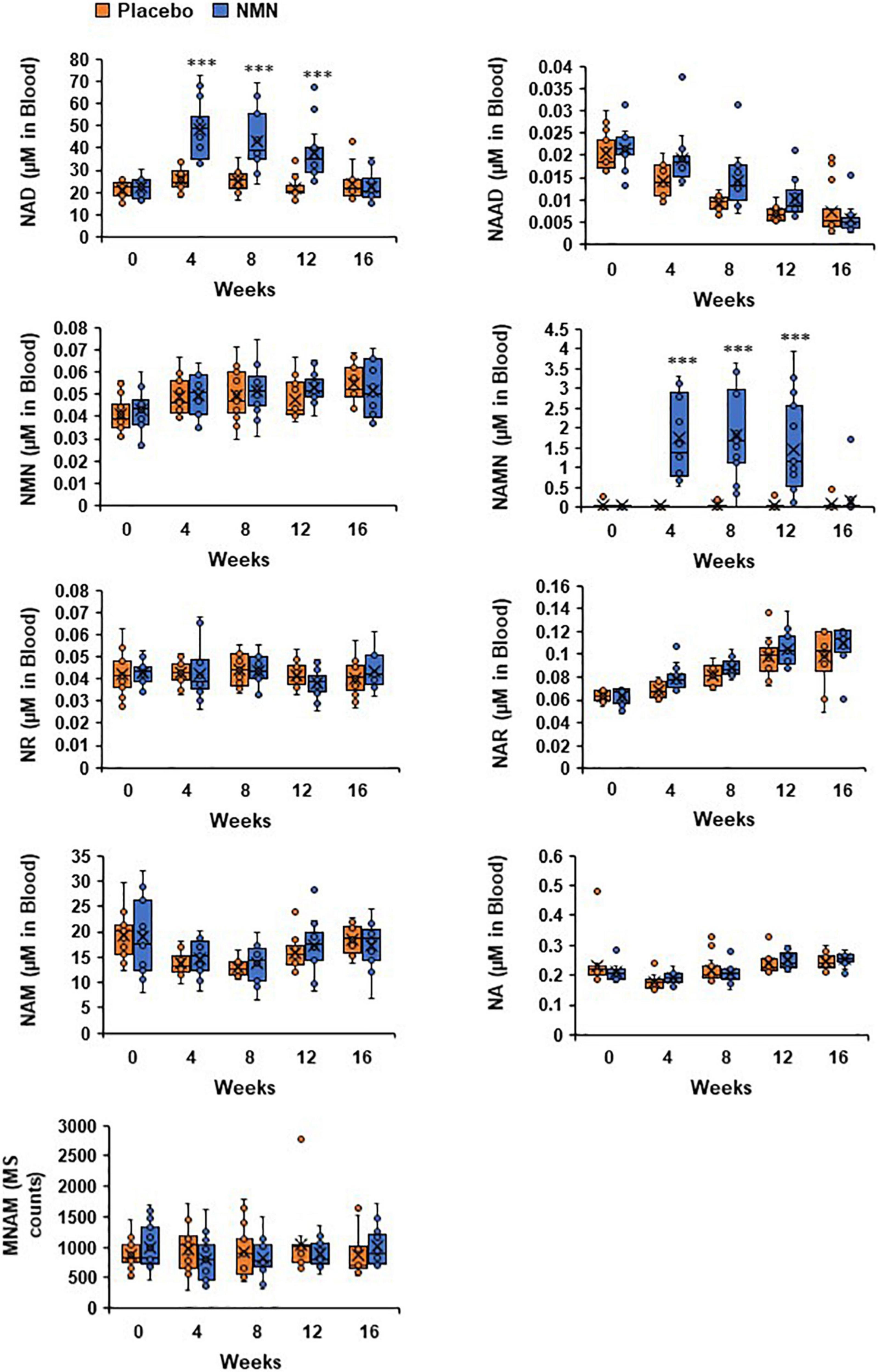
▲Levels of NAD+ and NAMN in blood were increased by oral administration of NMN. NAD metabolome in blood was measured every 4 weeks. NAD metabolome in each time point was showed in box plots. In the box plots, the median is indicated by the line within the boxes. The lower and upper boundaries of the boxes indicated 25th and 75th percentiles. The upper and lower lines above and below the boxes represent the whiskers. Orange boxes represent placebo group (n = 15 at 0, 4, 8 weeks, n = 14 at 12, 16 weeks) and blue boxes represents NMN group (n = 15). Levels of MNAM were calculated by integrating peak area of each chromatogram (MS counts). Three asterisks mean statistical significance: p-value < 0.001.
The primary outcome of this study was to assess the safety of NMN oral administration. No abnormalities were observed in the laboratory data, including liver function markers, AST, ALT, and γ-GTP. Although high dosage of NA, around 2 g per day, are used for the treatment of dyslipidemia in clinical practice, the amount of NMN in this study was significantly smaller and not harmful to liver. Another study examined 500 mg of NMN administration at a single dosage, but no significant increase was observed in the levels of AST and ALT. As NMN is a nucleotide, we also examined the levels of uric acid (UA), and we found that they were stable during administration of NMN. Thus, NMN administration may not disturb UA metabolism at least in healthy people. NA is a drug for dyslipidemia that decreases LDL-cholesterol and triglyceride (TG) serum levels . However, the TG, LDL-cholesterol, HDL-cholesterol, and Total-cholesterol levels remained unchanged both in the placebo and the NMN groups. The subjects in this study were healthy volunteers, and the levels of these lipids were almost within normal range, and no significant changes were observed during NMN administration. Flushing is a commonly observed side-effect in NA-treated patients. In this study, no such complaint was observed in both the placebo and the NMN-treated groups. Only gastrointestinal symptoms were the observed adverse effects associated with this intervention in both placebo and NMN- treated groups.
One person treated with placebo was dropped off from the study at 8 weeks due to the continuous abdominal bloating. Although one participant treated with NMN also had a mild stomach discomfort right after taking the NMN, this symptom disappeared without any medications. In this study, the vaccination for COVID-19 was allowed. Two of six in the placebo group and six of seven in the NMN group subjects complained the adverse reactions, such as fever, joint pain, or fatigue, after the vaccination.
Although there might be the trend of higher incidence in the NMN group, the difference between the groups was not statistically significant (p = 0.10 in Fisher’s exact test). It is suggested that viral infection depleted the NAD+ levels and blunted the anti-viral effects. Thus, NMN administration increases NAD+ levels and may boost innate immunity during COVID-19 infection or vaccination. Taken together, we concluded that oral administration of 250 mg NMN for 12 weeks is safe and well-tolerated.
In this study, we administrated 250 mg of NMM per day by dividing to 125 mg twice in the morning and evening. In a previous animal study, NMN was administered at 300 mg/kg per day for 12 months. However, the increase in NAD+ levels in tissues was marginal. Although 250 mg/day is significantly less compared to the dosage administered in mice, considering dose conversion from animal to human studies (approximately 1.2 g/day), 250 mg of NMM per day was an adequate dosage to increase and sustain the NAD+ levels in blood. Furthermore, NMN was fed with drinking water or food in the animal studies, whereas human took NMN as tablets twice per day. Perhaps the difference in the ways of taking NMN may attribute to the elevation of NAD+ levels in blood.
Human clinical trials have shown that NR can boost NAD+ levels in peripheral blood mononuclear cells (PBMC) and whole blood. In addition, NAD+ levels in urine were also increased by NR administration. Thus, it has been widely shown that NR can increase NAD+ levels in human blood. In contrast, only one study has reported the effect of NMN on NAD+ levels in human subjects. This study reported the increase of NAD+ levels in PBMC after administration of NMN at 250 mg/day for 10 weeks . In line with the findings of that study, our study also demonstrated that administration of NMN at 250 mg/day could significantly increase NAD+ levels in the whole blood. In addition, our study revealed the time course change of NAD+ levels. Although NAD+ levels were significantly increased at 12 weeks, the highest level was observed at 4 weeks. It is possible that an adaptation to higher NAD+ levels had occurred, and certain NAD synthesis enzymes, such as Nmnat, were downregulated. It is important to investigate whether longer NMN administrations periods (greater than 1 year) can still sustain increased NAD+ levels.
We also observed the drastic increase in NAMN levels after administration of NMN. The significant increase in deamidated NAD+-related metabolites, such as NAAD and NAR, has also been observed in other studies that administered NR to human subjects. It has been reported that orally administered NAM was converted to NA through deamidation by intestinal microbiota, and was absorbed from large intestine as NA. Recently, we demonstrated that orally administered NR was cleaved to NAM by BST1 followed by conversion to NA by microbi. The absorbed NA contributed to NAD+ synthesis through the Preiss-Handler pathway generating NAMN as an intermediate. Therefore, it is possible that orally administered NMN is also converted to NA by microbiota in human subjects. Indeed, both NR and NMN were reported to fail to increase NAD+ levels in skeletal muscles, which lack the Preiss-Handler pathway. It is very important to clarify the metabolic pathways of orally administered NMN in humans in order to maximize the efficiency of NMN supplementations.
It is clearly demonstrated that NMN can significantly increase NAD+ levels in whole blood, but the extent of this increase may vary among individuals. Thus, we analyzed the correlations between individual parameters and the increased amount of NAD+ levels after NMN administration. Because we used a fixed dosage of NMN for all participants in this study, the body weight and age were assumed as correlated factors. However, physical parameters, such as body weight and body composition, revealed a weak correlation. In contrast, pulse rate exhibited a strong positive correlation with the increase in NAD+ levels. The exact reason is unclear, but pulse rate may be related to energy expenditure, and it is possible that pulse rate is a confounding factor of energy expenditure. However, further studies are necessary for the precise interpretation of this result. Several blood parameters, such as Hb, Na, LD, Total ketone, HbA1c, TG, and HDL, also showed a weak correlation with the increase in NAD+ levels. Among these parameters, ALT showed the highest correlation. Liver is the most important organ to generate NAD+ in humans. In particular, deamidated NAD synthesis pathways, such as de novo and Preiss-Handler pathway, were most active in the liver. In our study, we observed the significant rise in NAMN levels, an intermediate of deamidated NAD synthesis pathway. It is possible that certain liver functions may correlate with NAD synthesis after NMN oral administration.
The present study demonstrated that oral NMN administration of 250 mg/day can significantly increase and sustain the levels of NAD+ in whole blood at 4-weeks until the end of administration without any apparent adverse effects. In addition, we found that the HR is strongly correlated with the increase in NAD+. Thus, oral administration of NMN can be a practical strategy to boost NAD+ levels in humans.
Article from:Okabe K, Yaku K, Uchida Y, Fukamizu Y, Sato T, Sakurai T, Tobe K and Nakagawa T (2022) Oral Administration of Nicotinamide Mononucleotide Is Safe and Efficiently Increases Blood Nicotinamide Adenine Dinucleotide Levels in Healthy Subjects. Front. Nutr. 9:868640. doi: 10.3389/fnut.2022.868640
Supplier Introduction: China glutathione supplier and NMN manufacturer GSHworld, the company mainly develops biotechnology and industrialization. As a global pioneer in enzymatic catalytic ATP regeneration technology, our company advocates green production and is committed to providing customers with better and more environmentally friendly products and services. Glutathione Manufacturer,NMN Factory,Citicoline Sodium supplier,China NMN manufacturers
PREVIOUS:Leaders Of Anqing City Visited Anhui GSH BIO-TECHNOLOGY CO.,LTD.
NEXT:New Study: NMN Supplementation Improves Muscle Strength in Older Healthy Men
+86-755-23577295
+86 18718790084
Room 832, Building 12, Shenzhen Bay Science and Technology Ecological Park, Yuehai Street, Nanshan District, Shenzhen China